On December 7, Sen. Ron Johnson (R-Wis.) held the latest of several forums with medical, research, and legal professionals to discuss COVID-19. A passionate advocate for Americans injured by the COVID-19 vaccine, Johnson’s actions to try and bring issues related to COVID-19 treatment are admirable. Unfortunately, he is a lone warrior, and Johnson’s activities to expose the flaws and possibly even wrongdoing in the federal agencies’ response to the pandemic were even used against him.
As promised, this is the first of a series of articles breaking down the information shared in the three-hour panel discussion titled “COVID-19 Vaccines: What They Are, How They Work, and Possible Causes of Injuries.” All the panel participants are considered dissident physicians, researchers, and attorneys. Some of them are dissident because they advocate and use repurposed medications to treat COVID. Others highlight COVID vaccine side effects or oppose their use at all. Still others criticize the medical and research establishment and point to what they see as corruption. Some do all three.
Still, they are some of the most credentialed physicians, researchers, and accomplished litigators on the planet. Attorney Aaron Siri was one of the first to speak. The information he shared is from a program called v-safe, run by the CDC. While you have probably heard of the Vaccine Adverse Event Reporting System (VAERS), v-safe is less well-known.
V-safe was explicitly developed to monitor patients’ experience following the COVID vaccines but was also used for the monkeypox vaccine rollout. It is a smartphone-based system used according to the CDC’s protocol as “an active surveillance program to monitor the safety of COVID-19 vaccines during the period when the vaccines are authorized for use under Food and Drug Administration (FDA) Emergency Use Authorization (EUA) and possibly early after vaccine licensure.”
“The purpose of v-safe surveillance is to rapidly characterize the safety profile of COVID-19 vaccines when given outside a clinical trial setting and to detect and evaluate clinically important adverse events and safety issues that might impact policy or regulatory decisions,” the document reads.
It also explains:
“However, even large clinical trials, like the COVID-19 vaccine clinical trials that are enrolling tens of thousands of volunteers, might not be large enough to detect rare adverse events (for example, those occurring at rates of <1 per 100,000 people vaccinated). Furthermore, for some clinical trials of COVID-19 vaccines, the follow-up period to monitor for possible adverse events with delayed onset may not be completed for all subjects prior to issuance of an EUA or licensure. Additionally, exclusion criteria for clinical trials may limit generalizability of safety and efficacy findings to special populations, such as those with certain chronic illnesses or pregnant women.”
To summarize, the clinical trials were not concluded when the FDA issued the EUA and were not large enough to detect all side effects. Additionally, people with specific conditions were excluded from the trials, such as pregnant women and people who had recovered from a documented COVID infection. Therefore, according to the CDC at the time, “robust post-authorization safety monitoring of COVID-19 vaccines is a public health priority.”
Recommended: People of the Lie
Then, the entire population, including pregnant women and the COVID-recovered, essentially became the largest clinical trial ever recorded. The data set covers December 2020 to August 2022. Most of the more than 10 million vaccine recipients registered in the system during the initial vaccine push between January and May 2021.
Yet in order for physicians and the public to access this data, Siri had to sue on behalf of the Informed Consent Action Network (ICAN). When the not-for-profit received the information, it was loaded into a database for the public to view.
As Siri points out in his testimony, the v-safe data has some advantages over VAERS and clinical trials. First, it is a much larger sample size than a clinical trial. Second, it is not filtered by the vaccine manufacturer like most trial data is. Finally, while VAERS and v-safe collect adverse side effects, the denominator is available in v-safe to determine accurate occurrence rates, and the information is standardized.
According to Siri, v-safe recorded two types of data. First, it asked vaccinated individuals if they were experiencing a predetermined set of reactions, such as fever and pain at the injection site. According to Siri, this list is what the CDC tells vaccinated individuals to expect as part of the body mounting the desired immune response to the vaccine. Participants are asked about these symptoms for a week after any dose of vaccine.
What v-safe did not collect in any standardized way were severe side effects, such as myocarditis, pericarditis, and transverse myelitis. Siri noted. “It would have been an incredible opportunity to gather that information in a systematic way.” Then Siri showed a slide from a CDC presentation from October 2020 that listed these three severe adverse reactions and several dozen more as “AEs of Special Interest.” AE stands for adverse events. “All the issues we now know with the vaccine, that we know the vaccine can cause, are not listed there on check-the-box options,” he pointed out.
Recommended: Head of Twitter’s Censorship Team Shadowbanning Conservatives Worked for Both FBI and CIA
The other type of data v-safe collects is called health impact data. It asks vaccine recipients if the symptoms they experienced made them unable to engage in everyday activities, miss work or school, or seek a doctor’s care. If they sought healthcare, recipients stated whether it was telehealth, a doctor’s office, the emergency room, or hospital admission. Here is what that data showed:
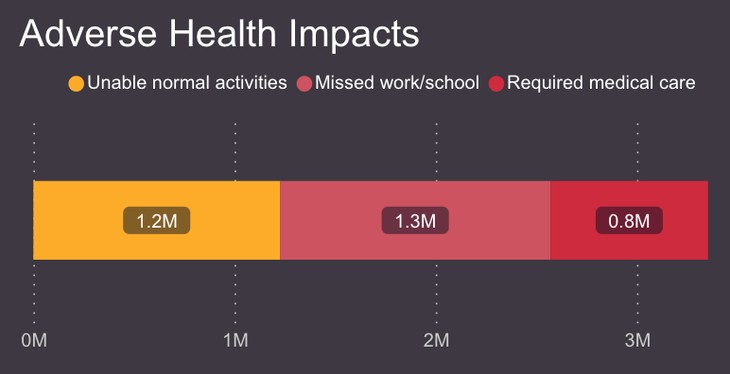
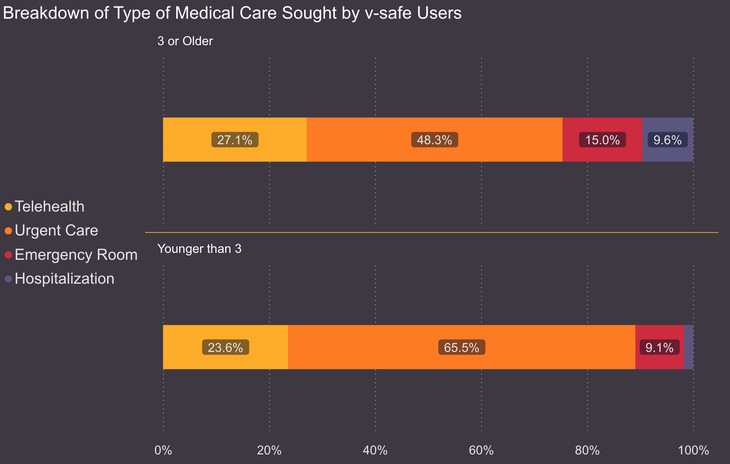
Approximately 8% of vaccine recipients, or one in 13, according to Siri, required medical care after receiving the vaccine. Of those over age three, about one in eight, or 71,911, were hospitalized. So, what questions need to be answered if the goal is accountability for public health officials? First, why were the severe adverse events not included in the standardized data collection by the CDC? The stated purpose of the v-safe system was to collect data that the size or duration of a clinical trial might not catch.
Second, as Siri asked, what was the threshold to end the program? The rate at which participants required medical care and hospitalization appears alarming. What was an acceptable rate? Someone from the v-safe needs to answer. Next, where is the free-form response data? Vaccine recipients were asked whether they wanted to report any other symptoms or conditions with a response box. The CDC did not turn over the data from that to ICAN.
Finally, why did it take a court ruling to make this data accessible to physicians and patients? A population-wide experiment was underway, and patients were owed all available information before consenting to an experimental therapy. In the protocol, the agency anticipated sharing this data.
Data from v-safe will be important in the beginning phases of the COVID-19 vaccination program. Regular updates will be provided to advisory committees and data review groups. It is anticipated that v-safe data will be shared with the scientific community and with the public through manuscripts and public reports.
Why have many Americans ever heard of v-safe? How come they don’t know that more than 20% of vaccine recipients miss school or work, and one in 13 seek medical care? Accountability demands answers. Up next, some shocking numbers our public health “experts” are ignoring.