On Wednesday, the CDC’s Advisory Committee on Immunization Practices (ACIP) voted unanimously to add the COVID-19 mRNA vaccines to the Vaccines for Children (VFC) program. Addition to the VFC list requires the government to pay for these vaccines if a patient can’t afford them in order to “ensure access.” As an added bonus, the vaccine manufacturers will remain free from liability for vaccine injuries because the product is on the VFC list. The government will still pay for vaccines, and the manufacturers will be held harmless. It would be fair to call this vote the “Pfizer and Moderna continuing profit plan.”
While the CDC tried desperately to separate the VFC from the vaccination schedule and back away from eventual vaccine mandates during Wednesday’s discussion, its rhetoric was absurd. No vaccine will be added to the VFC list if the agency does not believe ACIP will place it on the schedule. Why would the government pay for a vaccine that isn’t recommended?
Wednesday’s vote essentially set up a performative vote on Thursday to add the COVID-19 vaccines to the vaccination schedules for all Americans. The CDC has been updating recommendations for the COVID-19 vaccines since December 2020, when they launched. Its website already recommended the mRNA vaccines for all Americans over six months.
In fact, the CDC issued an interim immunization schedule for the COVID-19 vaccine for all Americans over six months old on Monday. The vaccine schedules proposed to ACIP mirror the interim ones. ACIP previously recommended COVID vaccines and boosters for various populations. It should surprise no one that ACIP voted unanimously again on Thursday to add them to the immunization schedules for children and adolescents.
Physicians and state governments rely on the vaccine schedule. Doctors use it to recommend vaccines for patients. Some states defer to the schedule to create vaccine mandates for school attendance and sports participation. Before the addition of the COVID vaccines, the immunization schedule for children from birth to six years old looked like this:
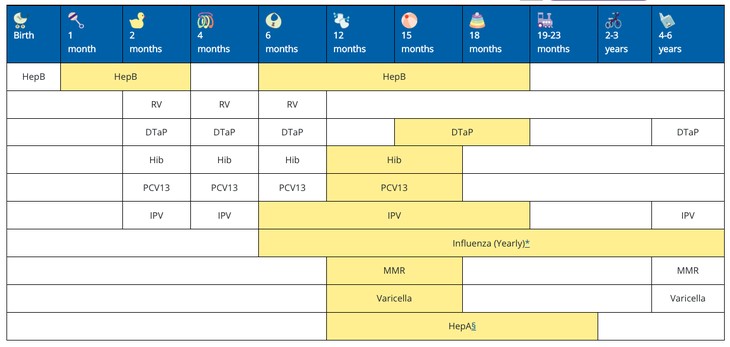
Following the vote Thursday, the CDC will add three doses of an mRNA COVID vaccine for children under six to the schedule. Details on dosage and timing for both COVID vaccine brands, different age groups, and health statuses will be added to the appropriate vaccine schedule.
However, it is all a semantic game. Most physicians follow the CDC recommendations when they are made. While the vote updates the schedule for everyone, the only real change once ACIP approves the vaccine schedules is the number of states that automatically incorporate the new vaccines into school attendance and sports participation requirements.
According to an analysis by the University of Illinois, Chicago, laws in 31 states and Washington D.C. require that children receive age-appropriate immunizations according to the schedule recommended by ACIP. Some also require boosters in later grades — the ones marked with a triangle reference ACIP.
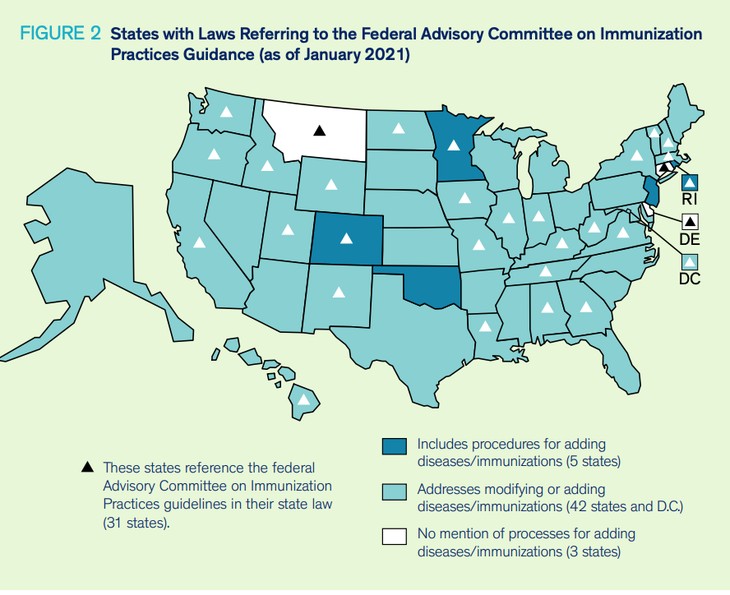
Seven states’ laws reference the American Academy of Pediatrics (AAP), while ten states’ laws discuss FDA approval. The AAP followed the CDC recommendations for COVID-19 vaccines before ACIP added them to the schedule. All COVID-19 vaccines currently used in the United States are under an emergency use authorization (EUA). If a state law references FDA approval, the current Pfizer and Moderna vaccines do not meet that standard.
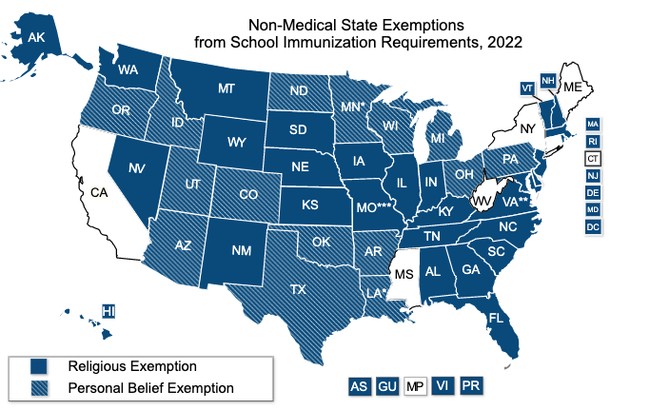
States also differ in their management of exemptions. All 50 states and Washington D.C. allow medical exemptions, though the requirements vary from state to state. Only medical exemptions are allowed in California, Connecticut, Maine, Mississippi, New York, and West Virginia. The remaining states and D.C. all allow religious exemptions with varying requirements. Fifteen states also offer a personal belief exemption, giving parents the most flexibility in vaccinating their children.
The addition of the COVID-19 vaccine to the immunization schedule for children may break the lock on blind implementation of the ACIP recommendations. The CDC changed the definition of a vaccine after it became apparent that the mRNA jabs did not prevent illness or transmission. The current products are also offered under a EUA. Every state must decide if its legislature can take ACIP recommendations seriously under these unprecedented conditions.
As Dr. Paul Offit, a member of the FDA’s vaccine advisory committee, noted about the COVID vaccines, “Well, in some ways, it’s a matter of your style. In other words, some parents may argue, well, if there’s any benefit, then I’ll accept what I think is a low risk. And others may argue, well, if there’s any risk, I don’t want to risk that if the benefit is so low. So I think — I just don’t think it’s going to make much of an impact in otherwise healthy young people.” An increasing number of parents are seeking educational freedom. It should not surprise lawmakers if the same constituency begins to demand medical freedom.









Join the conversation as a VIP Member