As the opioid crisis rages on throughout the country, an FDA panel has made a recommendation that could help everyday Americans fight opioid overdoses.
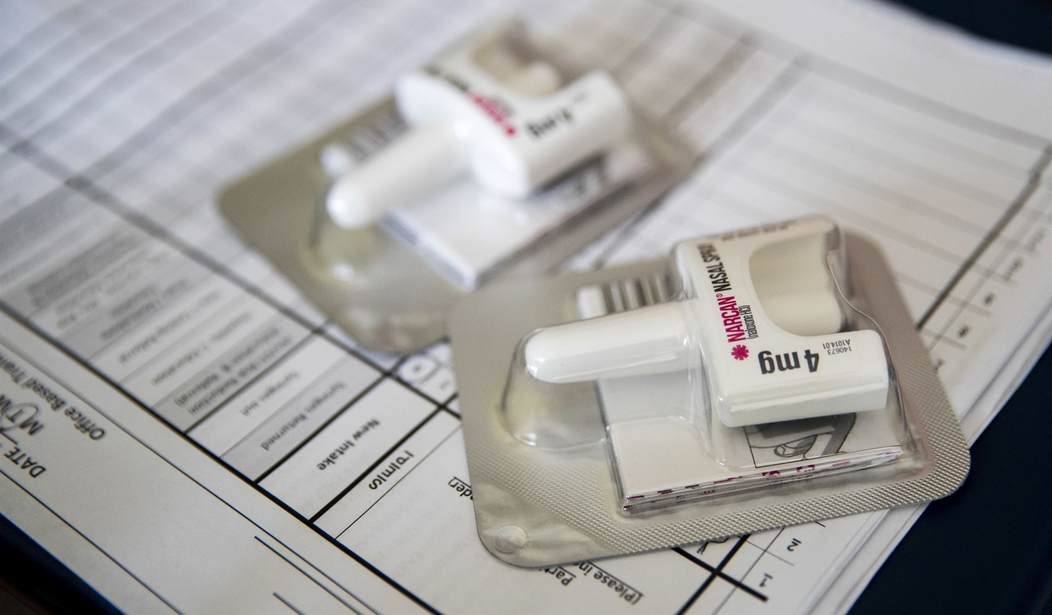
The panel recommended this week that the overdose-reversing medication naloxone, best known as Narcan but also available under other band names, be made available over the counter.
As it stands now, Narcan is readily available in pharmacies in all 50 states. Pharmacists can sell the drug to anyone who requests it, but experts say that there are some issues with that arrangement. First, the instructions for the use of Narcan can be confusing, and second, the stigma surrounding drug use may make some people less likely to approach a pharmacy counter to ask for Narcan.
Related: Mexico Is Not Really an American Friend
The panel’s recommendation would make it easier for anyone who needs Narcan to access it anonymously in drugstores, grocery stores, and even from vending machines.
“We believe that nonprescription naloxone may help address these barriers,” said Dr. Jody Green of the FDA.
The panel wants Narcan’s manufacturer, Emergent Biosolutions, to revise the instructions and make the packaging less confusing. Emergent has said that it will address the issues with the packaging even as it insists that focus group users were able to successfully navigate the directions.
“Emergent said it plans to move all the directions to a single panel and add pictograms, per FDA’s suggestion,” reports the Associated Press.
Even before Emergent can address the issue with instructions, the panel recommends that the FDA fast-track making Narcan more readily available to consumers.
“There’s perhaps a far greater risk of delaying the availability of the product given the climate of this crisis and its devastating consequences,” said Maria Coyle, the panel’s chair and a pharmacy professor from Ohio State University.
The panel’s vote isn’t binding, but the recommendation was unanimous among its members.
The FDA will take the panel’s recommendation under consideration, but community advocates take encouragement from the vote. Helping remove the stigma of Narcan and making it more easily accessible can only help curb overdoses.
“It’s going to make a tremendous impact on how people view the medication,” said Sheila Vakharia, deputy director of research and academic engagement at the Drug Policy Alliance, to the AP. “It will help to destigmatize it and make people know it’s safe and easy to use.”
It’ll be interesting to see what the FDA does next.









Join the conversation as a VIP Member